Fixation of coral spats with resin for synchrotron radiation imaging (BAMline)
Using the EpoFix Resin and following the EMS instructions for the preparation of the resin.
Ten days old spats of the coral Stylophora pistillata were fixed with resin for synchrotron radiation imaging (BAMline) at BESSY II in Berlin.
Samples preparation
- Take one filtered tip (20 µl) and cryotube (2ml) per each sample. Cut the top part of the tip (this is where the spat will be placed) and the bottom part of the tip.
- Fit the bottom part of the tip in the lid of the cryotube. If necessary cut more of the bottom-tip to ease the interlock.
- Mix 15 parts of resin with 2 parts of hardener in a 2 ml tube. At this point bubbles will form, so use a vacuum system to remove them.
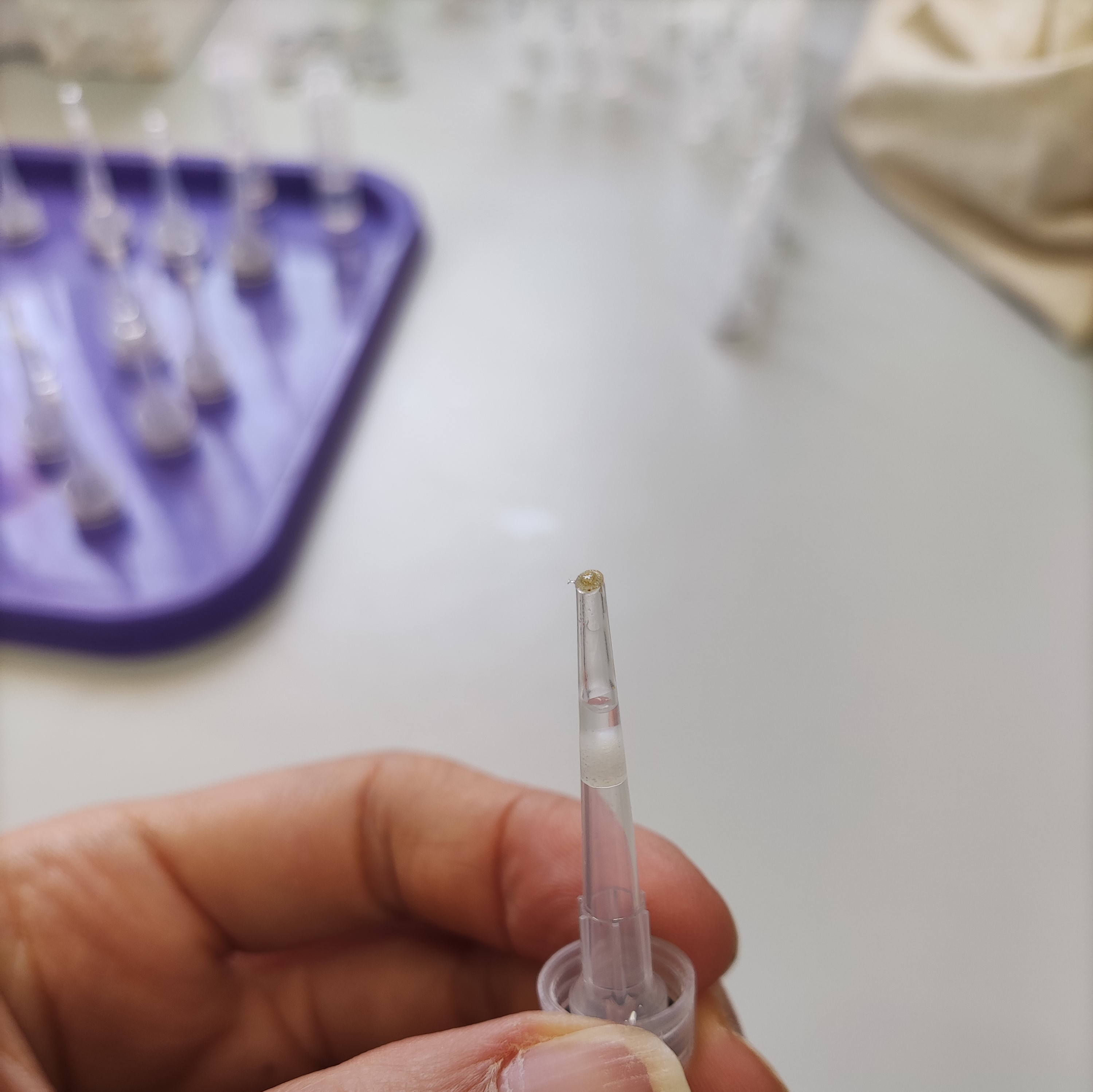
- Put a drop of resin mix at the top of the tip using a Pasteur pipette.
- Gently place the coral spat at the top of the tip using a razor blade.
- Gently add a drop of resin mix on the coral spat, wait for the resin to drip down the pipette. Add a second drop.
- After 1 hour add a third drop, make sure that the resin doesn’t drip down (the resin is almost solidified at this point).
- After 24 hours insert the tip + cup into the cryotube and close it. Your samples are ready for synchrotron radiation imaging!